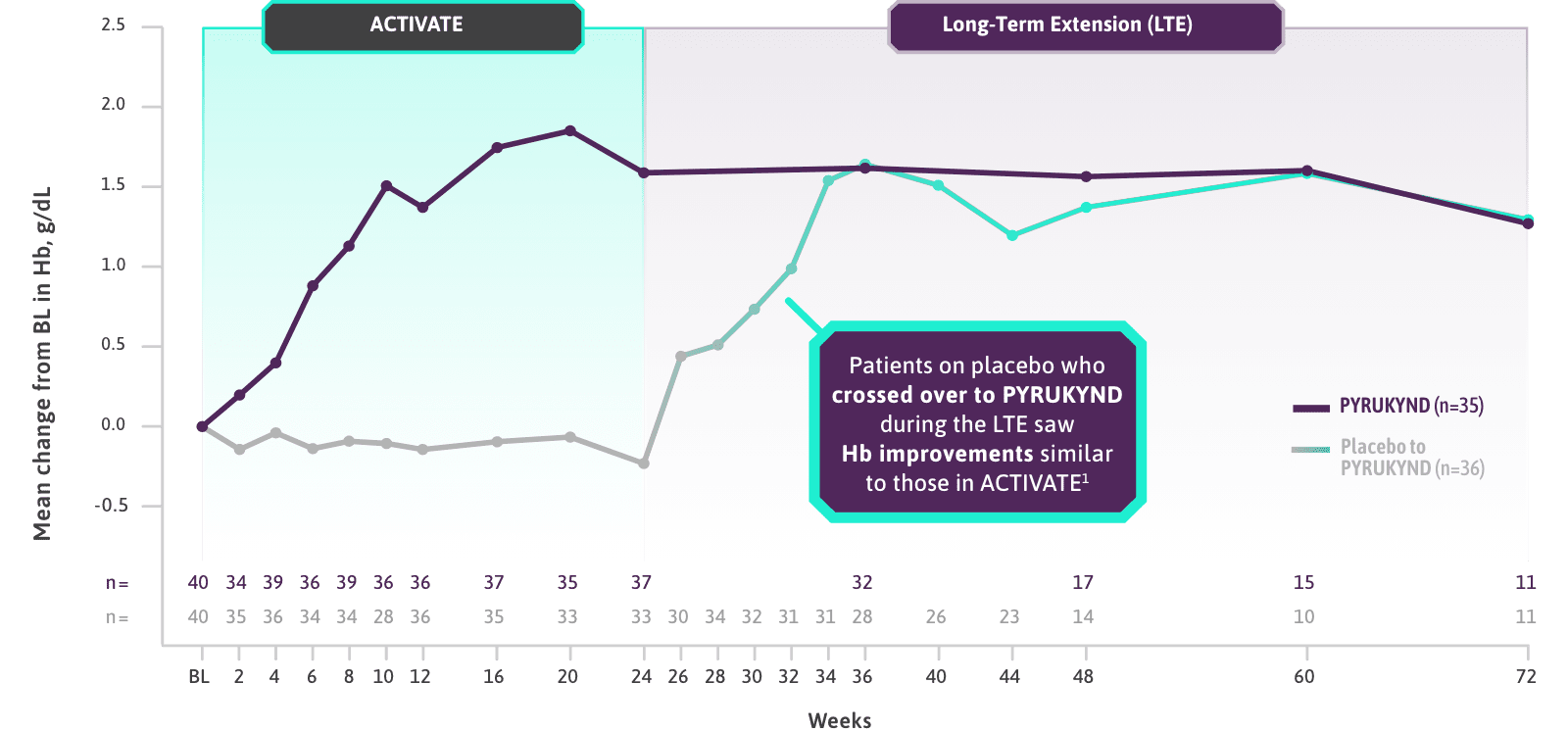
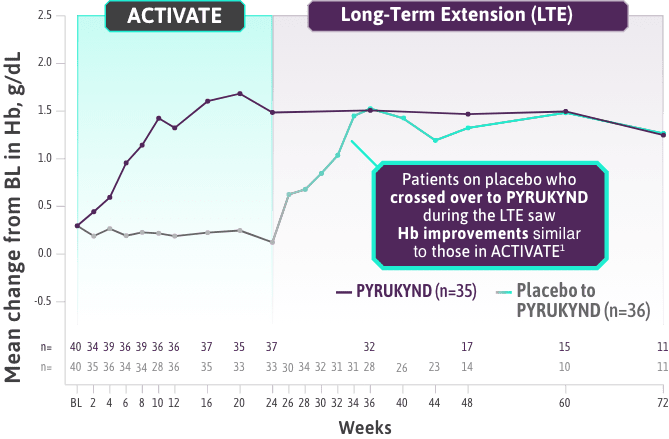
ACTIVATE: IN PATIENTS WHO WERE NOT REGULARLY TRANSFUSED
PYRUKYND® (mitapivat) delivered a significant, clinically meaningful increase in Hb1
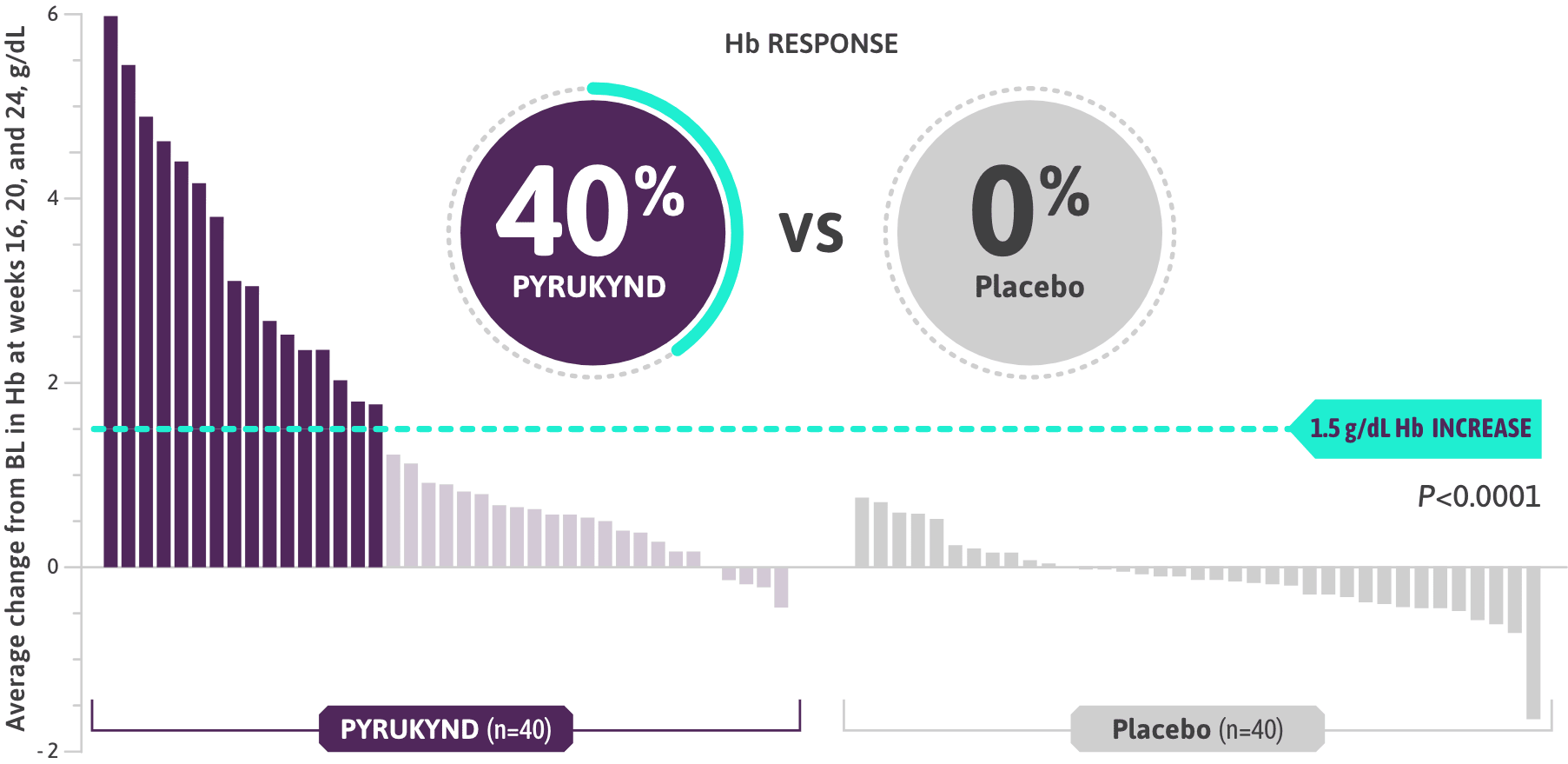
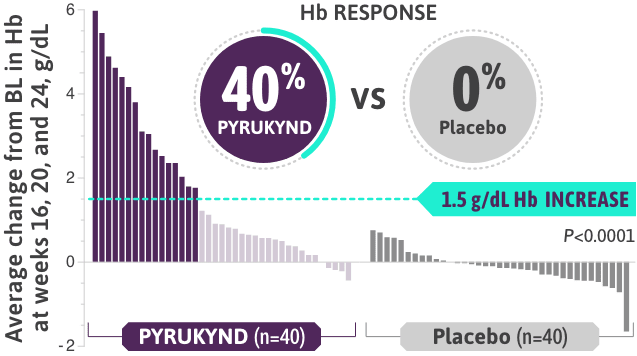
- Clinically meaningful Hb response (≥1.5 g/dL increase from baseline) in ≥2 assessments over the 12-week fixed-dose period (weeks 16, 20, and 24 without transfusions)1
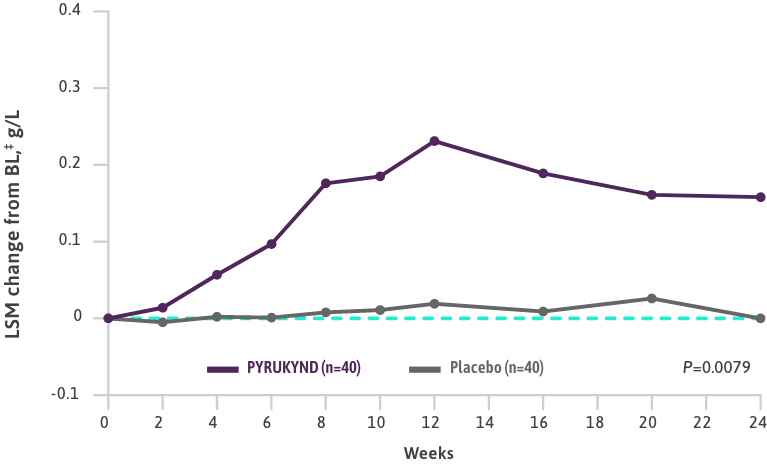
- The majority of patients on PYRUKYND saw an increase in Hb, while the majority on placebo saw a decrease in Hb (weeks 16, 20, and 24)1*
*Approximately 99% of all randomized patients completed 24 weeks of treatment.
Patients on PYRUKYND experienced improvements in tiredness and shortness of breath, which are symptoms of pyruvate kinase (PK) deficiency1†
†Difference in LS Mean of PYRUKYND minus placebo (scale 0–10): -1.1, SE 0.4 for tiredness and -0.3, SE 0.3 for shortness of breath, as collected in the daily Pyruvate Kinase Deficiency Diary where lower scores represent a lower disease burden.1
 Hear from Dr Fertrin
Hear from Dr Fertrin
Clinical manifestations and a treatment option for PK deficiency
Kleber Yotsumoto Fertrin, MD, PhD
Director, Sickle Cell Disease and Iron Overload Program
Seattle Cancer Care Alliance
Assistant Professor
University of Washington School of Medicine
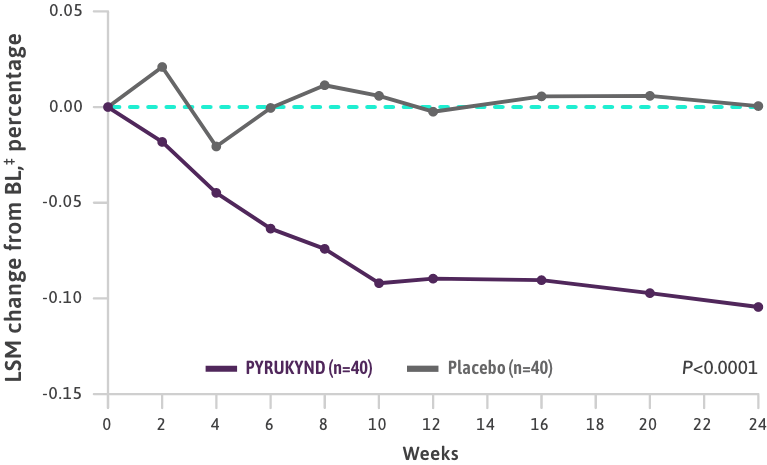
THE PK DEFICIENCY DIARY (PKDD) TRACKED CHANGES IN SIGNS AND SYMPTOMS OF HEMOLYSIS AND ANEMIA
Patients taking PYRUKYND measured changes in major signs and symptoms of PK deficiency, such as:
- Lower Score=Less Severe
JAUNDICE
Scale: 1-4
TIREDNESS
Scale: 1-10
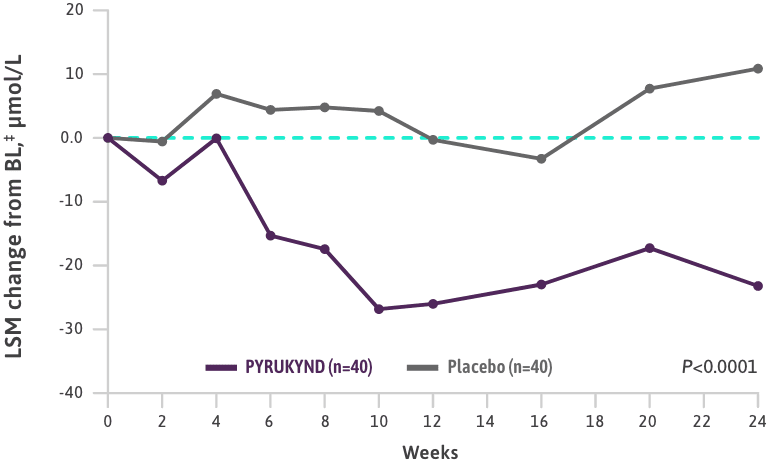
Patients on PYRUKYND saw improvements in jaundice, a sign of hemolysis1§
‡Baseline was defined as the average of all screening assessments within 45 (42 + 3) days before randomization for subjects randomized and not dosed or before start of study treatment for subjects randomized and dosed.2
§For jaundice symptom change, the difference in LSM of PYRUKYND minus placebo (scale 0–4): -0.4, SE: 0.1 as collected in the daily Pyruvate Kinase Deficiency Diary where lower scores represent a lower disease burden.1
ACTIVATE CLINICAL STUDY DETAILS
In patients who were not regularly transfused
A 24-week, randomized, placebo-controlled clinical study1
PYRUKYND has been studied for up to 5 years in multiple clinical studies in 155 participants1,4
STUDY DESIGN1-3
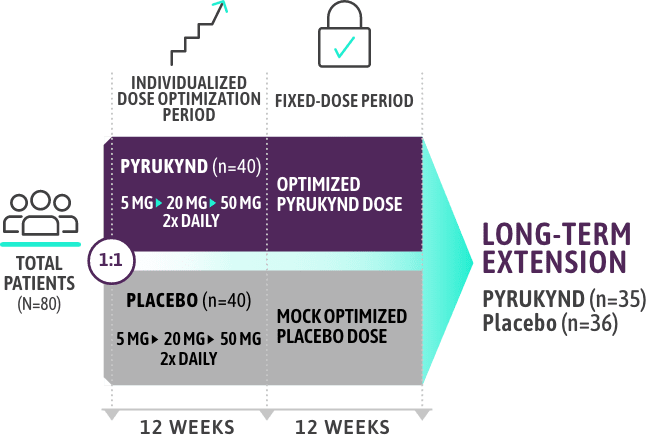
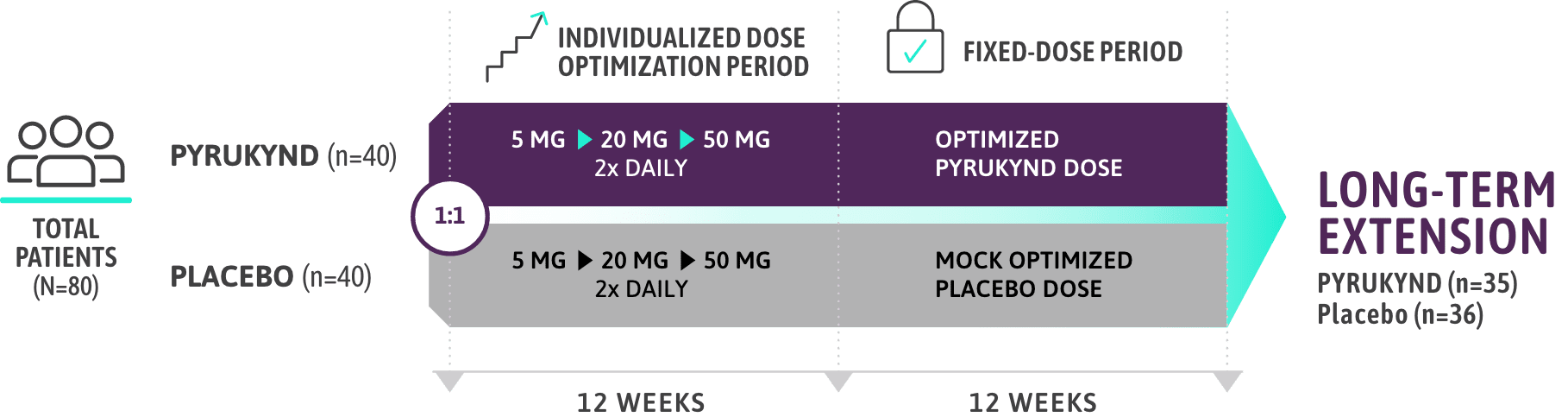
KEY ELIGIBILITY CRITERIA
- Presence of at least 2 variant alleles in the PKLR gene, of which at least 1 was a missense variant1
- ≤4 transfusions in the 52-week period prior to treatment and 0 transfusions in the 3-month period prior to study1
- Patients who were homozygous for the c.1436G>A (p.R479H) variant or had 2 non-missense variants (without the presence of another missense variant) in the PKLR gene were excluded1
KEY EFFICACY ENDPOINTS
- Clinically meaningful Hb response (≥1.5 g/dL increase from baseline) in ≥2 assessments over the 12-week fixed-dose period (weeks 16, 20, and 24)2
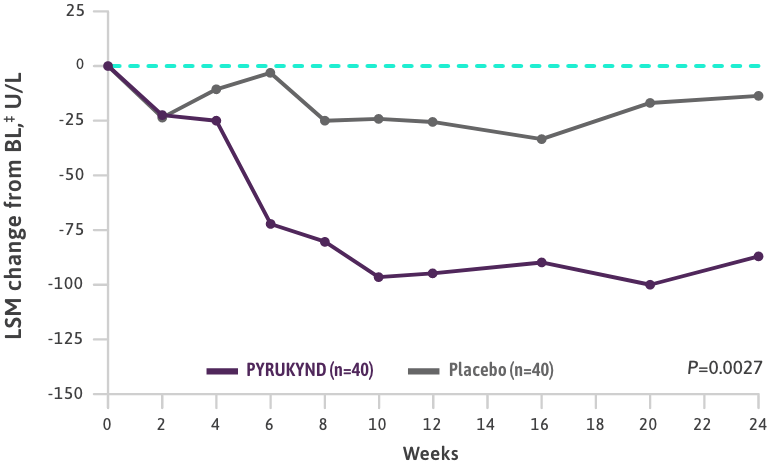
- The ACTIVATE trial also evaluated the average change from baseline (weeks 16, 20, and 24) for Hb, indirect bilirubin, reticulocytes, LDH, haptoglobin, and changes in major symptoms of hemolysis and anemia2
LONG-TERM EXTENSION STUDY
- The long-term extension study is ongoing with Hb response data currently out to 19.5 months3
Baseline characteristics reflect a broad range of patients with PK deficiency1
PATIENT DEMOGRAPHICS
- The median patient age was 33 years (range: 18-78 years)
- 55 patients (69%) had the missense/missense PKLR gene variant, and 25 patients (31%) had the missense/non-missense PKLR gene variant
- 58 patients (73%) had a history of splenectomy
HEMOGLOBIN (g/dL)
- The median baseline Hb was 8.5 g/dL (range: 6.4-10.2 g/dL)
EVIDENCE OF COMPLICATIONS & COMORBIDITIES ASSOCIATED WITH PK DEFICIENCY
- Iron overload (median baseline ferritin was 479 ng/mL [range: 21-5890 ng/mL])
- Chelation therapy use in the year before the first dose of study treatment in 15 patients (19%)
- Decreased bone mineral density in 64 patients (80%) who had a baseline femoral neck or lumbar spine T-score <-1.0
- History of cholecystectomy in 58 patients (73%)
The presence of iron overload and low bone density reflects a high disease burden1,5,6
BL=baseline; Hb=hemoglobin; LDH (U/L)=lactate dehydrogenase units per liter; LSM=least squares mean; QOL=quality of life; SE=standard error.
References: 1. PYRUKYND. Prescribing information. Agios Pharmaceuticals, Inc.; 2022. 2. Data on file. Agios Pharmaceuticals, Inc. 3. Grace RF, Glenthøj A, Barcellini W, et al. Durability of hemoglobin response and reduction in transfusion burden is maintained over time in patients with pyruvate kinase deficiency treated with mitapivat in a long-term extension study. Presented at: American Society of Hematology Annual Meeting; December 11-14, 2021; Atlanta, GA. https://ash.confex.com/ash/2021/webprogram/Paper147711.html. Accessed January 9, 2022. 4. Al-Samkari H, Grace RF, Glenthoej A, et al. Bone mineral density remains stable in pyruvate kinase deficiency patients receiving long-term treatment with mitapivat. Presented at: American Society of Hematology Annual Meeting; December 11-14, 2021; Atlanta, GA. 5. Grace RF, Bianchi P, van Beers EJ, et al. Clinical spectrum of pyruvate kinase deficiency: data from the Pyruvate Kinase Deficiency Natural History Study. Blood. 2018;131(20):2183-2192. 6. Bianchi P, Fermo E, Glader B, et al. Addressing the diagnostic gaps in pyruvate kinase deficiency: consensus recommendations on the diagnosis of pyruvate kinase deficiency. Am J Hematol. 2019;94(1):149-161 [supplementary online material].
Indication
PYRUKYND is a pyruvate kinase activator indicated for the treatment of hemolytic anemia in adults with pyruvate kinase (PK) deficiency.
Important Safety Information
Acute Hemolysis: Acute hemolysis with subsequent anemia has been observed following abrupt interruption or discontinuation of PYRUKYND in a dose-ranging study. Avoid abruptly discontinuing PYRUKYND. Gradually taper the dose of PYRUKYND to discontinue treatment if possible. When discontinuing treatment, monitor patients for signs of acute hemolysis and anemia including jaundice, scleral icterus, dark urine, dizziness, confusion, fatigue, or shortness of breath.
Adverse Reactions: Serious adverse reactions occurred in 10% of patients receiving PYRUKYND in the ACTIVATE trial, including atrial fibrillation, gastroenteritis, rib fracture, and musculoskeletal pain, each of which occurred in 1 patient. In the ACTIVATE trial, the most common adverse reactions including laboratory abnormalities (≥10%) in patients with PK deficiency were estrone decreased (males), increased urate, back pain, estradiol decreased (males), and arthralgia.
Drug Interactions:
- Strong CYP3A Inhibitors and Inducers: Avoid concomitant use.
- Moderate CYP3A Inhibitors: Do not titrate PYRUKYND beyond 20 mg twice daily.
- Moderate CYP3A Inducers: Consider alternatives that are not moderate inducers. If there are no alternatives, adjust PYRUKYND dosage.
- Sensitive CYP3A, CYP2B6, CYP2C Substrates Including Hormonal Contraceptives: Avoid concomitant use with substrates that have narrow therapeutic index.
- UGT1A1 Substrates: Avoid concomitant use with substrates that have narrow therapeutic index.
- P-gp Substrates: Avoid concomitant use with substrates that have narrow therapeutic index.
Hepatic Impairment: Avoid use of PYRUKYND in patients with moderate and severe hepatic impairment.
Please see full Prescribing Information for PYRUKYND.